The BLISTER Score for CIED infection
Estimates the risk of CIED infection within 12 months of a procedure, and provides a threshold for which patients should be allocated an antimicrobial envelope.
About
INTRODUCTION:
Antimicrobial envelopes reduce the incidence of cardiac implantable electronic device (CIED) infections, but their cost currently restricts routine use in the NHS. Risk scoring could help identify which patients would most benefit from this emerging technology. We examined the factors associated with CIED infection and derived a novel, point-of-care risk score (BLISTER). We tested the utility of BLISTER versus the existing PADIT score in both standard and high-risk external validation cohorts, and modelled the cost-utility of different BLISTER and PADIT score thresholds as gatekeepers for TYRXTM antimicrobial envelope allocation.
METHODS:
For the derivation cohort, data were extracted from 2016 to 2019 and included all de novo implants, generator changes and non-infected lead interventions for transvenous CIEDs at a UK tertiary centre (Centre 1). CIED infection was defined as hospitalisation for device infection within 12 months of a procedure. The BLISTER score (0-25) was composed using weighted points from beta coefficients in multivariate Cox regression. A standard-risk, external validation cohort comprised distinct, consecutive patients from Centre 1 (2019-2020) and a second UK tertiary centre (Centre 2), and a high-risk external validation cohort comprised distinct, consecutive patients from Centre 1 (2020-2021) and a third UK tertiary centre (Centre 3). Cost-utility modelling was informed by analysis of inpatient stays, standardised tariffs, extraction and device component costs, and quality adjusted life year (QALY) and efficacy data extrapolated from analysis of the WRAP-IT trial. A 10% random variability in risk, cost and efficacy was introduced for sensitivity analysis.
RESULTS:
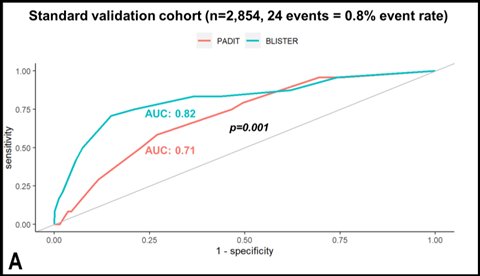
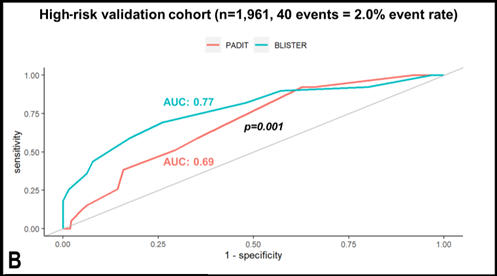
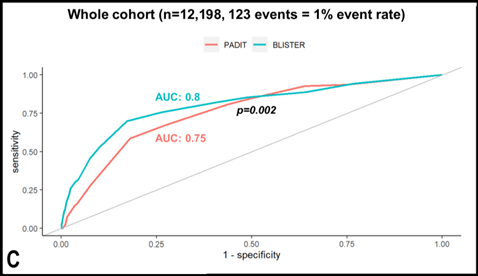
For derivation, 6,035 patients underwent 7,383 procedures; CIED infection occurred in 59 individuals (event rate: 0.8%). In addition to the constituents of the PADIT score, lead extraction (HR 3.3 (1.9-6.1), p<0.0001), C-reactive protein >50mg/l (HR 3.0 (1.4-6.4), p=0.005), re-intervention within two years (HR 10.1 (5.6-17.9), p<0.0001), and procedure duration over two hours (HR 2.6 (1.6-4.1), p=0.001) were independent predictors of infection. The BLISTER score demonstrated superior discriminative performance versus PADIT in the standard-risk validation cohort (n=2,854, event rate: 0.8%, AUC 0.82 vs 0.71, p=0.001), the high-risk validation cohort (n=1,961, event rate: 2.0%, AUC 0.77 vs 0.69, p=0.001) and in all patients (n=12,198, event rate: 1%, AUC 0.8 vs 0.75, p=0.002). In decision-analytic modelling of both scores, the optimum scenario assigned TYRXTM envelopes to all patients with BLISTER scores ≥ 6 (17.7%), delivering a significant reduction in infections (1% versus 0.7%, p=0.009) within the NICE cost-utility threshold (cost per QALY gained: £18,446).
CONCLUSIONS:
The novel BLISTER score was a valid predictor of CIED infection in a multi-centre analysis, and could facilitate cost-effective TYRXTM envelope allocation to selected high-risk patients.
Antimicrobial envelopes reduce the incidence of cardiac implantable electronic device (CIED) infections, but their cost currently restricts routine use in the NHS. Risk scoring could help identify which patients would most benefit from this emerging technology. We examined the factors associated with CIED infection and derived a novel, point-of-care risk score (BLISTER). We tested the utility of BLISTER versus the existing PADIT score in both standard and high-risk external validation cohorts, and modelled the cost-utility of different BLISTER and PADIT score thresholds as gatekeepers for TYRXTM antimicrobial envelope allocation.
METHODS:
For the derivation cohort, data were extracted from 2016 to 2019 and included all de novo implants, generator changes and non-infected lead interventions for transvenous CIEDs at a UK tertiary centre (Centre 1). CIED infection was defined as hospitalisation for device infection within 12 months of a procedure. The BLISTER score (0-25) was composed using weighted points from beta coefficients in multivariate Cox regression. A standard-risk, external validation cohort comprised distinct, consecutive patients from Centre 1 (2019-2020) and a second UK tertiary centre (Centre 2), and a high-risk external validation cohort comprised distinct, consecutive patients from Centre 1 (2020-2021) and a third UK tertiary centre (Centre 3). Cost-utility modelling was informed by analysis of inpatient stays, standardised tariffs, extraction and device component costs, and quality adjusted life year (QALY) and efficacy data extrapolated from analysis of the WRAP-IT trial. A 10% random variability in risk, cost and efficacy was introduced for sensitivity analysis.
RESULTS:
For derivation, 6,035 patients underwent 7,383 procedures; CIED infection occurred in 59 individuals (event rate: 0.8%). In addition to the constituents of the PADIT score, lead extraction (HR 3.3 (1.9-6.1), p<0.0001), C-reactive protein >50mg/l (HR 3.0 (1.4-6.4), p=0.005), re-intervention within two years (HR 10.1 (5.6-17.9), p<0.0001), and procedure duration over two hours (HR 2.6 (1.6-4.1), p=0.001) were independent predictors of infection. The BLISTER score demonstrated superior discriminative performance versus PADIT in the standard-risk validation cohort (n=2,854, event rate: 0.8%, AUC 0.82 vs 0.71, p=0.001), the high-risk validation cohort (n=1,961, event rate: 2.0%, AUC 0.77 vs 0.69, p=0.001) and in all patients (n=12,198, event rate: 1%, AUC 0.8 vs 0.75, p=0.002). In decision-analytic modelling of both scores, the optimum scenario assigned TYRXTM envelopes to all patients with BLISTER scores ≥ 6 (17.7%), delivering a significant reduction in infections (1% versus 0.7%, p=0.009) within the NICE cost-utility threshold (cost per QALY gained: £18,446).
CONCLUSIONS:
The novel BLISTER score was a valid predictor of CIED infection in a multi-centre analysis, and could facilitate cost-effective TYRXTM envelope allocation to selected high-risk patients.



References
E Maclean, C Monkhouse, et al.
European Journal of Arrhythmia & Electrophysiology. 2022;8(Suppl. 1):abstr98
Edd Maclean, Karishma Mahtani, et al.
Circulation. Arrhythmia and Electrophysiology 2024 January 23
The The BLISTER Score for CIED infection calculator is created by QxMD.
By using this site you acknowledge that you have read, understand, and agree to be bound by our terms of use and privacy policy. All content and tools are for educational use only, are not meant to be a substitute for professional advice and should not be used for medical diagnosis and/or medical treatment.